Hey Class!!
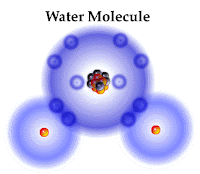
As we finish talking about the smallest particles that make up matter, we begin talking about the substances that are made from different combinations of atoms. A molecule is a combination of at least two atoms that form a strong chemical bond. A molecule might consist of a single element (O2) or of different elements (H2O).
BlogWork
Go to the following website: All About Molecules
Read the whole introduction to what a molecule is.
Then, pick one molecule and, in your own words, describe the molecule in a post.